14 Which of the Following Accurately Describes the Ph Scale
For example pH 4 is ten times more acidic than pH 5 and 100 times 10 times 10 more acidic than pH 6. B Scientists developed the geologic time scale.
A pH of 7 is neutral.

. The pH scale ranges from 0 to 14. The middle reading of 70 is considered to be neutral A reading below 70 is acidic while a reading of 70 or above is alkaline. The active site will become a catalyst.
Test the pH of everyday liquids such as coffee spit and soap to determine whether each is acidic basic or neutral. In a pH expression the hydronium ions H3O can be abbreviated simply as H. The greater the pH is the lower the acidity is.
If foreign strong substances dramatically change this pH our bodies can no longer function properly. Neutral solutions are. Which of the following statements about the pH scale is not true.
The pH scale is used to classify as acidic alkaline or neutral. PH is really a measure of the relative amount of free hydrogen and hydroxyl ions in the water. The scale ranges from 0-14.
The pH scale is used to measure acidity and alkalinity. A pH less than 7 is acidic. The pH of water is a measurement of how acidic or basic it is.
If pH is 8-14 is it Acidic Base Neural or neither. PH 0 has the highest hydrogen ion H concentration. The pH of water is an extremely essential indicator of water quality.
Most parts of our body excluding things like stomach acid measure around 72 and 76 on the pH scale a 7 is neutral on the scale. The pH scale runs from 0 most acidic to 14 neutral with 7 as an average acidity level. The pH scale runs from 0 most acidic to 14 most basic with 7 as a neutral.
The protein may change shape and. For each pH unit increase there is a 10-fold decrease in hydrogen ions. The range goes from 0 - 14 with 7 being neutral.
A solution with a pH of 4 has twice the H of a solution with a pH of 2. PHs of less than 7 indicate acidity whereas a pH of greater than 7 indicates a base. The pH scale is a number scale from 0 to 14.
The greater the pH is the lower the acidity is. The pH scale which measures from 0 to 14 provides an indication of just how acidic or basic a substance is. Well if you havent found the answer yet its D as the pH scale goes from 0-14 where 14 is the most alkaline and 0 is the most acidic.
The pH scale ranges from 0 to 14. Which of the following describes how the pH of the solution could change. Investigate how adding more of a liquid or diluting with water affects pH.
In a pH expression the hydronium ions H3O can be abbreviated simply as H. Which of the following is incorrect regarding the pH scale. PH is a measure of how acidicbasic water is.
Acidity is indicated by a pH less than 7 while a pH greater than 7 indicates a base. Litmus paper is an indicator used to tell if a substance is an acid or a base. The scale was invented in 1909 by a Danish biochemist called Søren Sørensen.
The greater the concentration of hydroxide ions the higher the acidity is. For each pH unit decrease there is a 10-fold decrease in hydrogen ions. PH is an abbreviation for potential hydrogen It is the measurement of hydrogen The pH scale ranges from 0 to 14.
The lower the pH reading the less oxygen is in the fluid you are testing whereas the. Chemistry Single Science Acids alkalis and salts. Question 10 1 pts If a mutation in DNA caused a new amino acid sequence to occur what might happen to the subsequent protein.
PH 14 has the highest hydroxide ion OH- concentration. The lower the pH is the more basic the solution is. 1 The recording method thats most useful in discovering the causes or results of a childs behavior is A.
Water that has more free hydrogen ions is acidic whereas water that has. It could increase from 40 to 70. It tells us how acidic or alkaline an.
For example Vinegar is an acid and measures 24 on the pH scale. A pH greater than 7 is basic. The range is 0 to 14 with 7 being the neutral value.
It describes how many hydrogen ions protons are present in a solution. When an acid is neutralised it forms a salt. The pH scale typically stretches from zero to 14 passing through a neutral pH7 freshly distilled water.
Which of the following statements about the pH scale is not true. The pH of water is an extremely essential indicator of water quality. A solution with a pH of 4 has twice the H of a solution with a pH of 2.
The colour of the paper matches up with the numbers on the pH scale to indicate what kind of substance is being tested. The lower the concentration of hydrogen ions the higher the acidity is. Therefore as you go up the scale the substance becomes less acidic.
Strong acids have a low pH while alkaline chemicals such as bleach and liquid drain cleaner have a high pH. The pH scale is logarithmic and as a result each whole pH value below 7 is ten times more acidic than the next higher value. It could decrease from 70 to 40.
A pH scale is a tool for measuring acids and bases. The greater the pH is the lower the acidity is. Which of the following accurately describes the pH scale.

The Ph Scale Chemistry For Non Majors
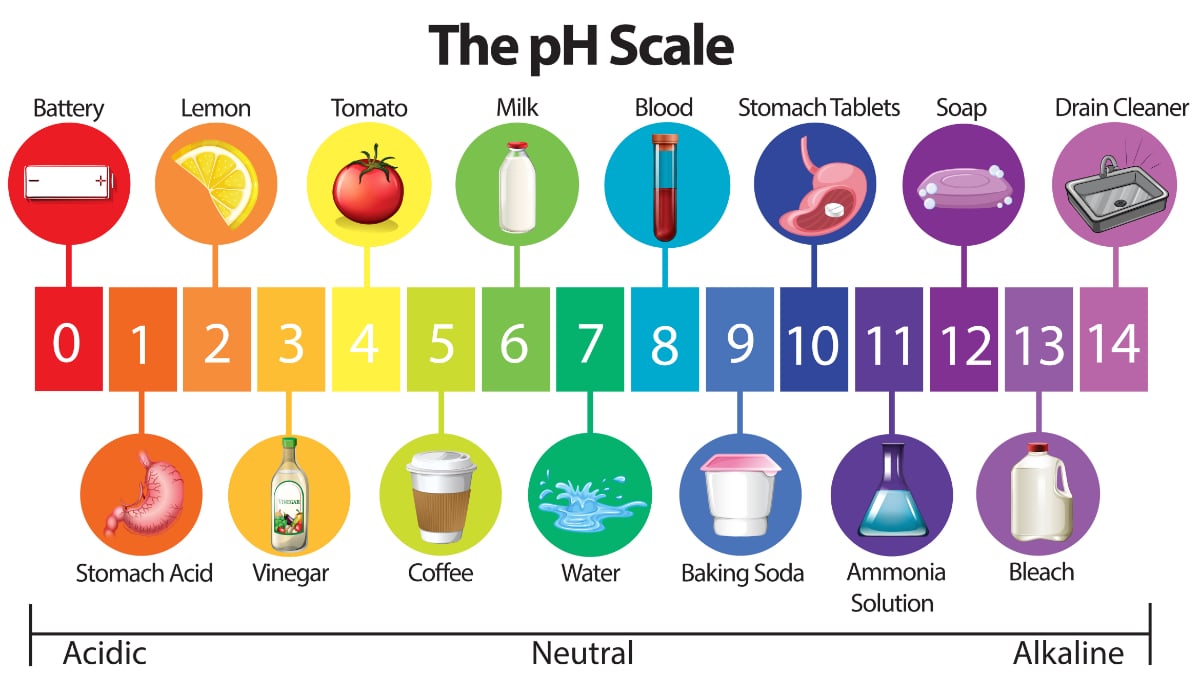
Water Quality 101 What Is Ph In Water Testing

The Ph Scale Of Common Chemicals Chemistry Notes Info Chemistry Experiments
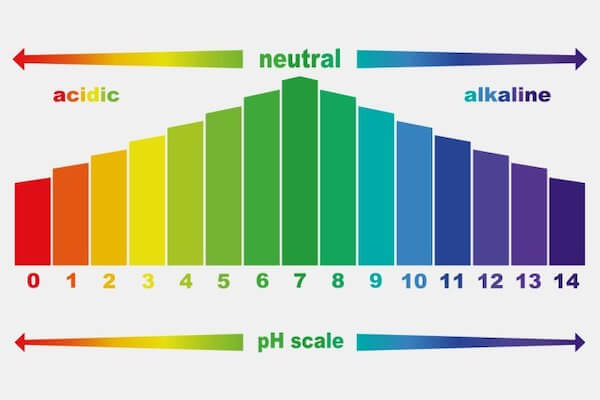
Understanding The Ph Scale Of Cleaning Chemicals And Why It Matters Corvus

Acidic Basic Neutral Solutions Determining Ph Video Lesson Transcript Study Com

Why Ph Value Is Measured Between 0 And 14 Pharmaceutical Guidelines
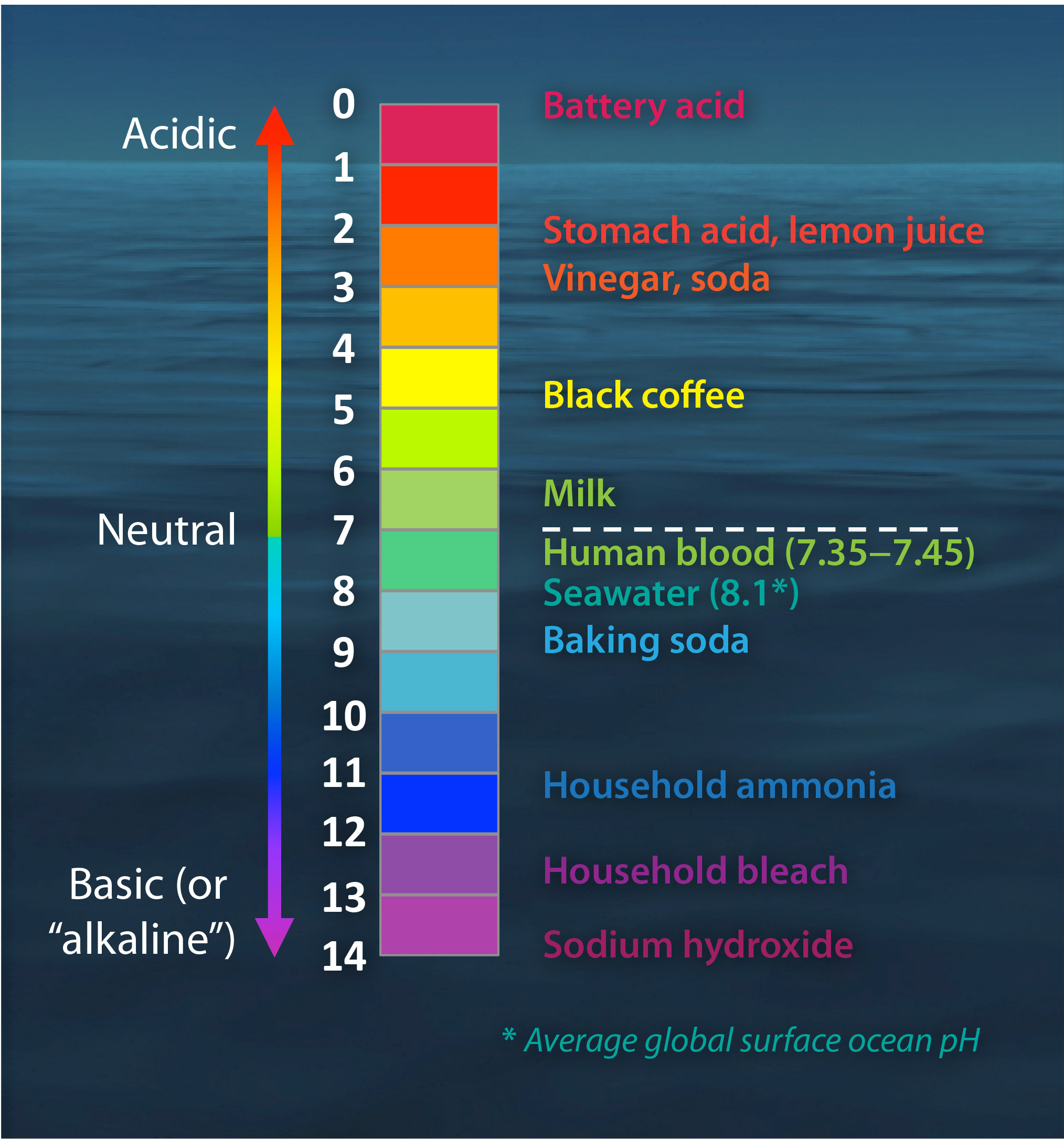
The Ph Scale With Some Common Examples

Ph Scale U S Geological Survey

Ph Scale In Simple Terms Youtube
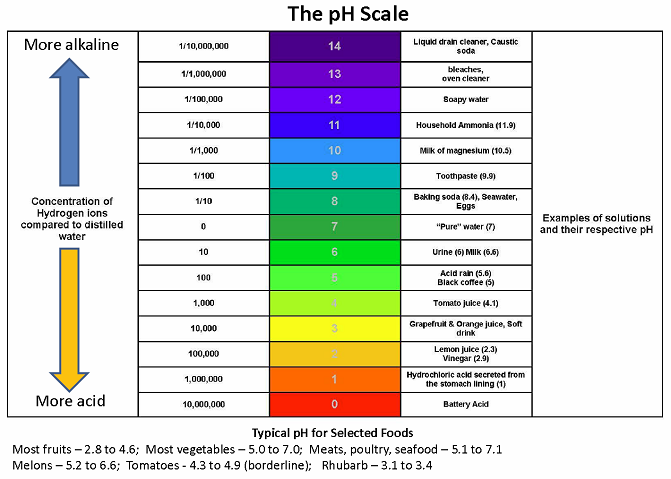
What Is Ph College Of Agriculture Forestry And Life Sciences Clemson University South Carolina
Ph Poh And The Ph Scale Article Khan Academy

Ph Scale U S Geological Survey

Alkaline Acidic Foods Chart The Ph Spectrum Acidic Food Chart Acidic And Alkaline Foods Health And Nutrition
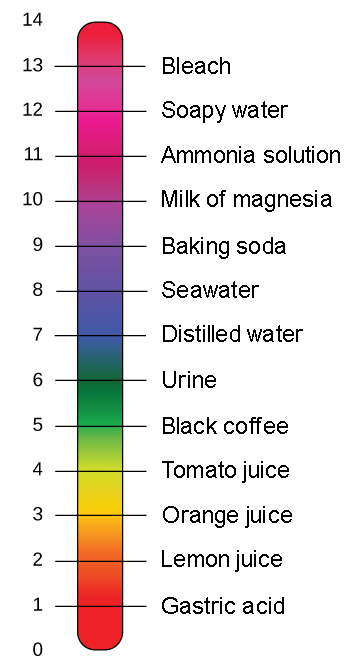
The Ph Scale Biology For Non Majors I

Ammonia Nh3 Is A Weak Base With A Kb Value Of 1 8 10 5 Dissociation Ammonia Base

Pin By Roshan On Ph Scale Remove Toxins Healthy Water Drinks Banner Printing
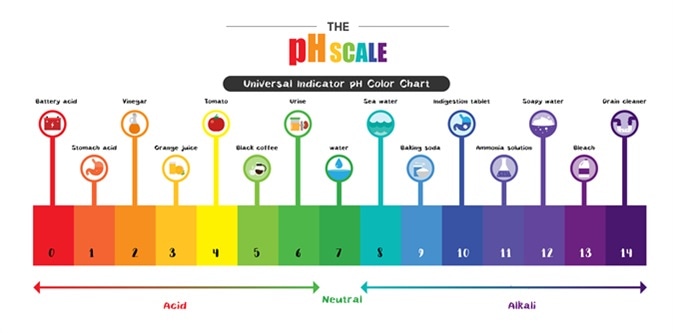



Comments
Post a Comment